
Business Type:Lab/Research institutions
Country: ![]() China (Mainland)
China (Mainland)
Business Type: Lab/Research institutions
Coreychem have done a lot of process optimization experiments can be stable production kg class, one hundred kilograms of product. Respectively, pantoprazole, Trametinib, cediranib and other new APIs.
Brief introduction
Pantoprazole is in a class of medications called proton-pump inhibitors. It works by decreasing the amount of acid made in the stomach. Pantoprazole binds irreversibly to H+K+ATPase (proton pumps) and suppresses the secretion of acid. As it binds irreversibly to the pumps, new pumps have to be made before acid production can be resumed. pantoprazole was highly specific binding only with the proton pump activity of 5,6 fragment, but also omeprazole in combination with inactive fragments 7-8, lansoprazole also with the non-active 7,8 and 3 piece binding fragment. duodenal and gastric ulcer healing rates using pantoprazole and omeprazole no significant difference, but pantoprazole better tolerated. For various causes gastric mucosal lesions induced upper gastrointestinal bleeding was no difference in the efficacy of omeprazole, adverse reactions are mild, treatment of upper gastrointestinal bleeding is one of the safety and reliable drug.
Synthetic route
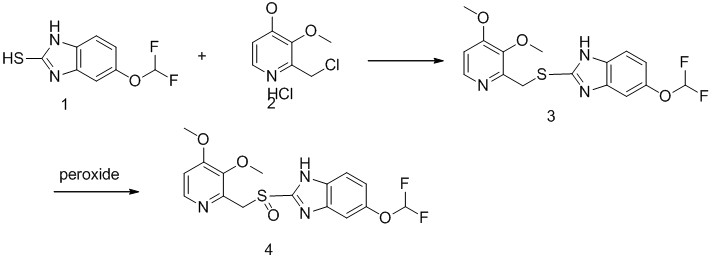
Synthesis description
The process for the preparation of pantoprazole involves condensation of thiol derivative 1 with
chloromethyl pyridine derivative 2 in the presence of inorganic base to yield 5-(difloromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl]thio-1H-benzimidazole 3, which upon further oxidation with a suitable oxidizing agent leads to the desired pantoprazole 1.
Product advantages
Our company optimized the first step reaction by screening the base application and adding a phase transfer catalyst to get good results, product 3 have a higher yields,and simple operation,we carried out industrial production. The second step Optimize the conditions for oxidation, by a good control of the process to reduce the double oxidative impurities, the double oxidation impurities can be controlled at less than 0.1% after purification.
Product parameters
CAS Registry Number :102625-70-7
Name:5-(DIFLUOROMETHOXY)-2-[[(3,4-DIMETHOXY-2-PYRIDINYL)METHYL]SULFINYL]-1H-BENZIMIDAZOLE
Molecular Weight 383.37
Value: 7.91±0.10 | Condition: Most Acidic Temp: 25 °C
Melting Point (Experimental) Value: 139-140 °C (decompose )
Other Names
5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl]sulfinyl]- (9CI) 1H-Benzimidazole,
(±)-Pantoprazole
2-[[(3,4-Dimethoxypyridin-2-yl)methyl]sulfinyl]-5-difluoromethoxy-1H-benzimidazole
5-(Difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole
5-(Difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole
Pantoprazole
Brief introduction
Trametinib (trade name Mekinist) is a cancer drug. It is a MEK inhibitor drug with anti-cancer activity.It inhibits MEK1 and MEK2.Trametinib had good results for metastatic melanoma carrying the BRAF V600E mutation in a phase III clinical trial. In this mutation, the amino acid valine (V) at position 600 within the BRAF gene has become replaced by glutamic acid (E) making the mutant BRAF gene constituitively active
Synthetic route
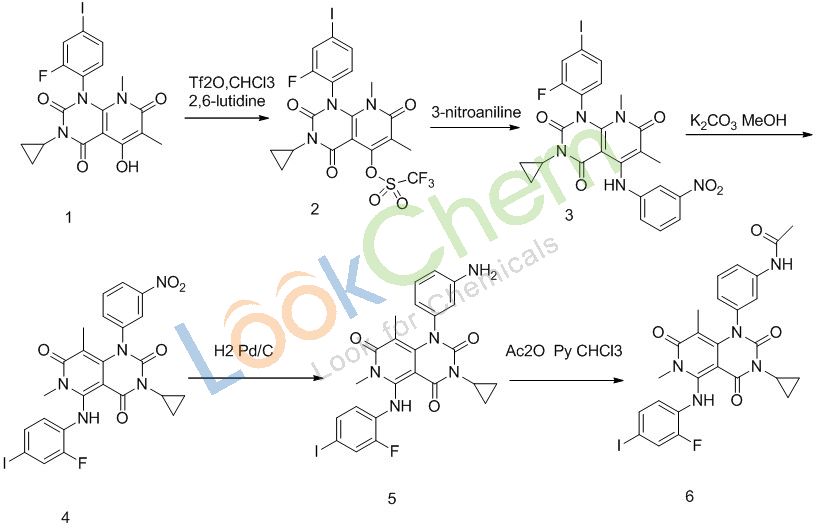
Synthesis description
1 is reacted with Tf2O by means of 2,6-lutidine in CHCl3 yielding 2, which is substituted with3-nitroanilline to afford 3. Subsequent rearrangement 3 with K2CO3, followed by reaction with hydrogen , leads to the 5, Trametinib is finally obtained by acetylization by means of Ac2O at CHCl3.
Product advantages
After each step is optimized to achieve the level of ten kilograms per batch production, mature technology, especially when the reduction catalyst is recycling in place, you can get a good economic effect.
Product parameters
CAS Registry Number 871700-17-3
Name:N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl]- Acetamide,
Molecular Weight 615.39
Melting Point :Value: 300-301 °C
Other Names
G 02442104
GSK 1120212
JTP 74057
Mekinist
N-[3-[3-Cyclopropyl-5-(2-fluoro-4-iodo-phenylamino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydro-2H-pyrido[4,3-d]pyrimidin-1-yl]phenyl]acetamide.